Every Patient Deserves a Safe Recovery.
Eliminate residual paralysis—simply and seamlessly
Every Patient Deserves a Safe Recovery.
Residual Paralysis is Common and Costly
The incidence of residual neuromuscular blockade is about 40%1, and remains as high as 10% even with universal sugammadex use2. A study at Temple University Hospital found that standardized TwitchView monitoring could significantly reduce postoperative pulmonary complications, yielding an estimated $4.6 million in annual cost savings—while requiring a technology investment of just 3.5% of that amount3.
Residual Paralysis is Common and Costly
The incidence of residual neuromuscular blockade is about 40%1, and remains as high as 10% even with universal sugammadex use2. A study at Temple University Hospital found that standardized TwitchView monitoring could significantly reduce postoperative pulmonary complications, yielding an estimated $4.6 million in annual cost savings—while requiring a technology investment of just 3.5% of that amount3.
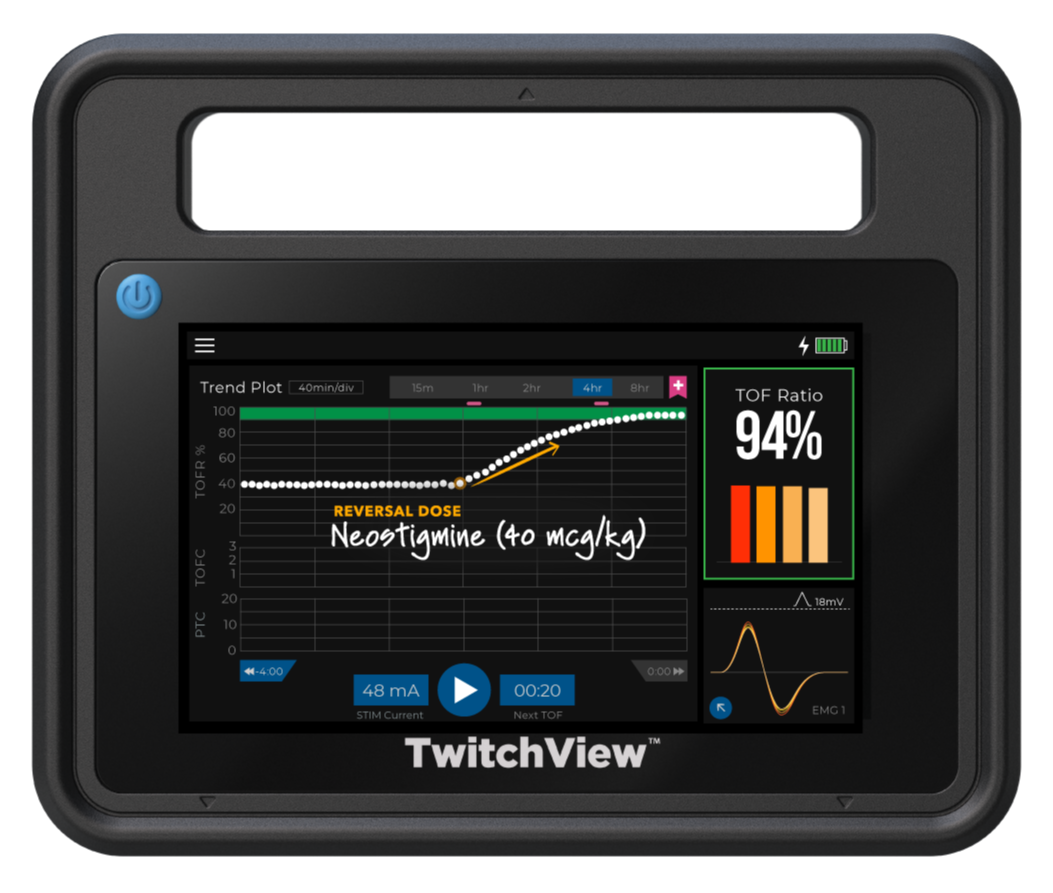
Residual Paralysis is Preventable
Residual paralysis is one of anesthesia’s most preventable risks. A landmark study using quantitative monitoring with TwitchView to guide the choice of reversal agent—neostigmine for moderate blocks and sugammadex for deeper blocks—and confirm full recovery prior to extubation eliminated residual paralysis and cut drug costs by 70%, saving $43 per patient compared to universal sugammadex use4.
Who We Serve
TwitchView transforms care—benefiting patients, clinicians, and the entire care team.
Patient
A safe recovery, free from residual weakness.
Anesthetist
Precision monitoring that integrates seamlessly into your workflow.
Peri-operative Team
Reduced PACU times and fewer postoperative complications.
Hospital
Reduced drug costs, compliance with ASA guidelines5, and higher quality of care.
Discover Your Hospital’s Savings with the TwitchView Train of Four Monitor
Answer a few quick questions, and we’ll prepare a customized report showing how much your hospital could save by reducing residual paralysis and optimizing reversal drug use. See your potential impact in dollars, tailored to your institution.
Residual Paralysis Stops Here.
See the TwitchView® TOF Monitor in action at your institution.
Residual Paralysis Stops Here.
See the TwitchView® TOF Monitor in action at your institution.